SomnusNooze
Awareness of disease means different things to different people. One’s awareness takes origin in experiences and goals, both personal and professional. Diversity in awareness therefore comprises our community of people who have hypersomnia, their significant others, healthcare professionals, and research scientists.
People who have hypersomnia need to become more self-aware of their symptoms; significant others, employers, and educators, more accepting of the limitations of the person with hypersomnia; and health care providers, more vigilant as to how hypersomnolence negatively impacts their patients’ health. Clinician scientists are becoming increasingly aware of unconventional treatments for hypersomnolence and are soon to launch clinical trials of even more novel medications. For the research scientist working at a laboratory bench, what form does awareness take? Their efforts are critical to leveraging the collective awareness of all of the stakeholders in hypersomnia if the diagnosis and treatment of hypersomnia are to be realized.
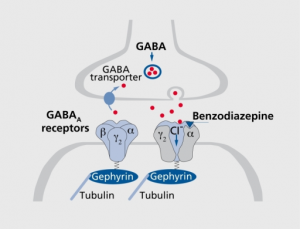
In this spirit, we would like to share where we find ourselves with the basic science of idiopathic hypersomnia (IH) and where this science might be headed in the immediate future. Although for many years IH has not attracted a considerable amount of attention from basic scientists, recent discoveries have yielded two successful grant applications funded by the National Institutes for Neurological Diseases & Stroke—a division of the National Institutes of Health (NIH), and, in turn, the Department of Health and Human Services. The most recent, and substantial (nearly $3 million spread over 4 years), is pending an award after regulatory approval to a team lead by Dr. David Rye at Emory University. (For further information, click HERE.) The funded work follows upon their findings published in 2012 in Science – Translational Medicine,1 in which they described their research regarding 14 people who had no sleep problems (i.e., control subjects) and 32 people who had idiopathic hypersomnia (IH) or Type 2 narcolepsy or were long sleepers with otherwise normal results on Multiple Sleep Latency Tests. The Emory researchers discovered a bioactive substance in the cerebrospinal fluid (CSF) of two thirds of the people with hypersomnia regardless of their formal diagnosis. This substance boosted the natural ability of the neurotransmitter gamma-aminobutyric acid (GABA) to dampen or inhibit the nervous system. The substance was not found in the CSF of any of the control subjects. Moreover, vigilance was improved in seven of seven subjects who exhibited this excess enhancement of GABA’s actions at the “A” subtype of GABA receptors (i.e., GABAAR) and who received the GABA antagonist flumazenil. Because researchers have not yet identified the exact constituent within the CSF of people with hypersomnia that enhances GABAAR function, some researchers and patients have come to affectionately refer to this substance simply as “sleepy juice.” Yet, there is much to be done to ultimately prove causality and thereby hasten the pace of progress in the realms of diagnosis and treatment.
In the late 20th century, Robert Koch, a German physician, who later received the 1905 Nobel Prize in Medicine, set forward four conditions that must be met to establish a microorganism as the causative agent of disease: (1) the microorganism must be found in all cases of the disease, (2) it must be isolated from the host, (3) it must reproduce the original disease when introduced into an unsuspecting host, and (4) it must be found or retrieved from the experimental host that is so infected. The Rye team has satisfied most of these points in demonstrating that (1) the GABA-ergic bioactivity (that is, the activity of the “sleepy juice”) is greater in people with hypersomnia than in control subjects,1 (2) the “sleepy juice” is likely a peptide with a fairly modest size (500-3,000 Daltons),1 (3) the amount of sleep time increases in rats in whom this component of CSF is infused into the central nervous system (unpublished data), and (4) antagonists of the GABAAR, such as flumazenil, have been shown to normalize vigilance in people with hypersomnia.1 Nonetheless, the magnitude of GABAAR enhancement by individual patients’ CSF samples in Rye’s initial study did not correlate with any measure of hypersomnolence, including flumazenil-related improvements in vigilance.
To “nail down” Koch’s postulates and thereby establish cause and effect (causality), Rye’s team will be performing new experiments. These experiments will compare GABAAR enhancement in people with IH and Type 2 narcolepsy with control subjects, people with Kleine-Levin syndrome, and people with obstructive sleep apnea in the hope of identifying a protein “fingerprint” that distinguishes the groups from one another. They will also look more closely at the CSF to see how and where the “sleepy juice” acts at submicroscopic levels by studying genetically engineered GABA receptors cloned into cell lines and, separately, mice bred to have genetically altered or missing GABA receptors.
The Rye team is at the doorstep of a major research project that seeks to identify the “sleepy juice” and how and why it works to promote hypersomnolence. The biological pathways responsible for its synthesis, accumulation, and degradation should then more rapidly identify themselves. This knowledge will breed awareness among basic researchers who are otherwise focused on how and why we sleep as opposed to treating disease. In turn, new knowledge will emerge, thereby identifying new means to diagnose hypersomnolence and a plethora of additional avenues for non-medicinal and medicinal means to improve the symptoms of hypersomnolence – even, ultimately, a cure.
1. Rye DB, Bliwise DL, Parker K, et al. Modulation of vigilance in the primary hypersomnias by endogenous enhancement of GABAA receptors. Sci Transl Med 2012;4(161):161ra151.