SomnusNooze
 Ægidius, Helene M, et al. “Pre-Treatment of Blood Samples Reveal Normal Blood Hypocretin/Orexin Signal in Narcolepsy Type 1.” Brain Communications, vol. 3, no. 2, 2021, doi:10.1093/braincomms/fcab050.
Ægidius, Helene M, et al. “Pre-Treatment of Blood Samples Reveal Normal Blood Hypocretin/Orexin Signal in Narcolepsy Type 1.” Brain Communications, vol. 3, no. 2, 2021, doi:10.1093/braincomms/fcab050.
The current gold standard diagnostic test to differentiate narcolepsy type 1 (NT1) from other hypersomnias is to measure the level of hypocretin in cerebrospinal fluid (CSF). The procedure to take a sample of CSF, called a spinal tap, is uncomfortable for the patient and not without risks. Researchers have tried to develop hypocretin blood tests in the past, but experts agreed that these tests weren’t accurate or consistent enough to be used in the diagnosis of NT1. A team of researchers in Denmark has been working on improving hypocretin blood testing, with the goal of having a highly accurate test with consistent, repeatable results.
As reported in the paper Pre-Treatment of Blood Samples Reveal Normal Blood Hypocretin/Orexin Signal in Narcolepsy Type 1 (Ægidius et al., Brain Commun, 2021), the research team first worked on testing several different methods of preparing blood samples for hypocretin testing. Hypocretin binds to protein carriers in blood. In order to detect just the hypocretin, it must be dissociated (or chemically unbound) from the protein carriers. After many trials, the team found that increasing the pH of the sample to 8 and heating it to 65-70°C provided the best results for this dissociation from protein carriers and therefore maximizing the detection of hypocretin.
A second round of research aimed to discover the best method for detecting hypocretin within the properly prepared samples (with increased pH and temperature). The test uses hypocretin antibodies. Three different hypocretin antibodies were tested, and although two (Phoenix and Pierce) detected similar levels of hypocretin, the third (Peninsula) detected a much higher level of hypocretin. It is possible that the Peninsula antibody detects a higher level because it binds to a different part of the hypocretin than the other antibodies.
Additional testing done in mice revealed another challenge—a high unspecific background signal. By analogy, this is like listening to the radio when there is a large amount of background static that drowns out the speaker’s voice. The researchers think that the background signal is likely caused by the antibodies that are supposed to bind to hypocretin binding to something else as well. The level of the background signal is very high, making it difficult to detect the actual hypocretin signal. The researchers attempted several methods to eliminate the background signal, but each method caused the hypocretin signal to disappear as well.
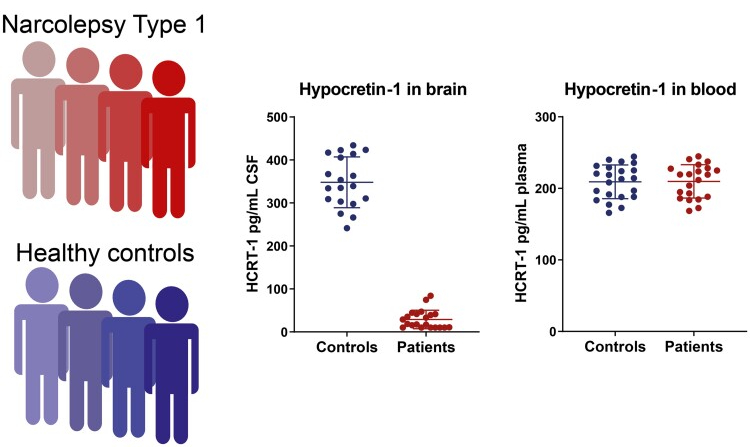
Finally, the research team applied their best methods to measure hypocretin levels in human blood samples. The narcolepsy type 1 patients and healthy controls, all of whom were from Denmark and Italy, provided both CSF and blood samples. While there was a clear difference in hypocretin levels in CSF (NT1 patients had much lower hypocretin levels than healthy controls), there was no difference in the level of hypocretin detected in the blood between the two groups. See image below.
 Ægidius, Helene M, et al. “Pre-Treatment of Blood Samples Reveal Normal Blood Hypocretin/Orexin Signal in Narcolepsy Type 1.” Brain Communications, vol. 3, no. 2, 2021, doi:10.1093/braincomms/fcab050.
Ægidius, Helene M, et al. “Pre-Treatment of Blood Samples Reveal Normal Blood Hypocretin/Orexin Signal in Narcolepsy Type 1.” Brain Communications, vol. 3, no. 2, 2021, doi:10.1093/braincomms/fcab050.
As it stands today, blood tests still cannot be used as a diagnostic tool to differentiate NT1 from other hypersomnias. However, this research does provide some interesting clues about the details of the autoimmune process that leads to NT1.
Why would people with NT1 have low hypocretin in CSF but normal levels in their blood? The data from this research suggest that blood hypocretin does not solely come from the central nervous system and that there could instead be a peripheral (non-CNS) source that contributes to the blood levels. Normal hypocretin blood levels in NT1 would mean that peripheral hypocretin-producing cells are not affected by the autoimmune attack that destroys the hypocretin-producing neurons in the brain (the hallmark of NT1 pathology). This implies that hypocretin itself is not the target of the autoimmune attack. Another protein found in the hypocretin-producing neurons (not the peripheral producers) would then be the primary autoimmune target, causing selective destruction of the CNS hypocretin-producing neurons (while leaving the peripheral producers safe from attack).
A different explanation for the normal blood hypocretin levels in people with NT1 could be that the hypocretin-producing neurons in the hypothalamus are more sensitive and prone to death following the autoimmune attack, whereas peripheral hypocretin-producing cells might recover more easily. This could result in low blood hypocretin levels at disease onset and then a recovery over time. If this turns out to be the case, then a low hypocretin blood level could help identify an active autoimmune process.
This research team has significantly improved testing methods and conducted important experiments on hypocretin in the blood. This provides a solid platform for future studies of blood hypocretin levels, which could help determine any possible function(s) of hypocretin outside of the CNS and any possible relevance of abnormal blood levels if and when they are seen (such as to identify other as yet unknown diseases or to further test the above-described hypothesis that low hypocretin blood levels could help identify an active autoimmune process in NT1). As a recognition of her important contributions, the primary author, Helene Ægidius, received the 2021 Young Scientist Award from the European Narcolepsy Network at their September 2021 conference. We hope to see her and others in the hypersomnia research community continue to study hypocretin in both the brain and body in hopes of future improvements in both diagnosis and treatment of narcolepsy and other hypersomnias.
Reviewed and approved by Gert Jan Lammers, MD, PhD, HF Medical Advisory Board member and primary author Helene Ægidius.
